Introduction to the In-Silico Trials Market
The in-silico trials sector is rapidly becoming a game-changing component of the life sciences and healthcare ecosystem. Using advanced computational modelling and simulation, organizations can design, test, and validate medical products before entering traditional clinical trials. Virtual patient models, artificial intelligence, and sophisticated algorithms are enabling researchers to predict safety, effectiveness, and product performance with greater precision. As healthcare providers push for faster innovation and cost optimization, simulation-driven research is gaining widespread adoption across pharmaceutical, biotech, and medical device industries.
Overview of the Digital Trial Simulation Market
Simulation-driven research is redefining how medical innovations are developed. By leveraging virtual patient modelling and artificial intelligence, organizations can assess product safety and performance long before traditional clinical testing begins. This shift is helping the healthcare industry reduce risk, increase efficiency, and accelerate innovation.
Growth Outlook and Market Expansion
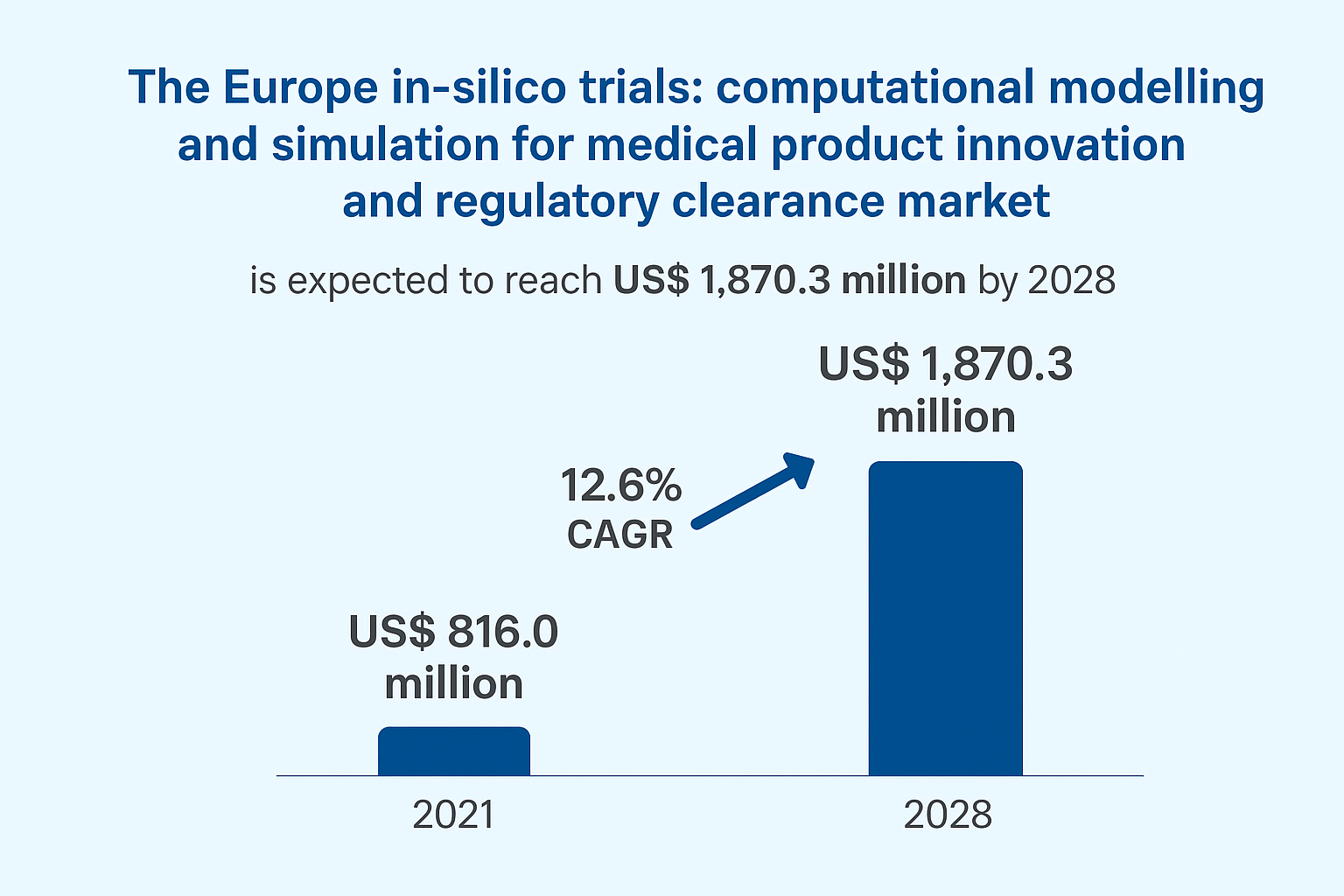
The market is set to rise from US$ 816.0 million in 2021 to US$ 1,870.3 million by 2028, registering a CAGR of 12.6%. Increasing demand for faster research outcomes, rising R&D costs, and expanding digital infrastructure are key drivers supporting this growth.
Sample Request PDF:
https://www.theinsightpartners.com/buy/TIPRE00039298
Efficiency Compared to Traditional Clinical Trials
Traditional trials are costly and slow, often limiting the scope of research. Simulation tools enable researchers to test multiple variables simultaneously, generating faster and more comprehensive insights. This efficiency significantly reduces development timelines and financial risk.
Increasing Role in Regulatory Approval
Simulation-based data is gaining recognition from regulatory authorities as valuable supporting evidence. As trust in computational models grows, digital trials are becoming an integral component of regulatory submissions.
Segmentation by Organization Size and Offerings
Both large enterprises and SMEs are adopting simulation tools. Large organizations benefit from extensive research resources, while smaller companies use digital platforms to remain competitive. Key offerings include products, platforms, and services tailored to diverse research needs.
Key Applications Fueling Demand
Virtual trials support discovery, development, pre-clinical research, and product evaluation. These capabilities enable organizations to optimize designs and predict outcomes earlier in the development process.
Expanding Clinical Applications
The technology is applied across multiple disease areas, including oncology, cardiovascular, infectious, and metabolic diseases. Its ability to simulate complex biological systems is driving broader adoption.
Regional Market Dynamics
Germany, the UK, France, Spain, and Italy lead the regional landscape due to strong healthcare investment and research capabilities.
Leading Industry Participants
• InSilicoTrials Technologies
• Feops
• CADFEM Medical GmbH
• Dassault Systèmes SE
• Virtonomy GmbH
• Certara Inc.
• Computational Life
• NOVA
• TwInsight Medical
• Ansys Inc.
Future Outlook
With continuous advances in AI and computing power, simulation-based research will become increasingly accurate and widely accepted. Collaboration across the healthcare ecosystem will play a crucial role in driving future growth.